Epitaxial growth of Ni(OH)2 nanoclusters on MoS2 nanosheets for enhanced alkaline hydrogen evolution reaction†
Abstract
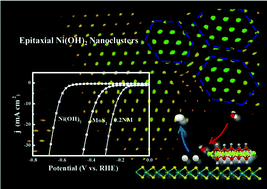
Constructing heterostructures is an effective strategy for designing efficient electrocatalysts. MoS2 is a star catalyst for hydrogen evolution reaction (HER) in acidic media; however, the alkaline HER activity is deficient due to the sluggish water dissociation process. Herein, Ni(OH)2/MoS2 heterostructures with Ni(OH)2 nanoclusters epitaxially decorated on the surface of MoS2 are synthesized towards the alkaline HER. As compared with MoS2, the epitaxial Ni(OH)2/MoS2 heterostructures show significantly enhanced HER activity in 1 M KOH, and the overpotential is decreased by nearly 150 mV to reach a current density of 10 mA cm−2. The substantial increase in turnover frequency (TOF) demonstrates that the intrinsic activity is greatly improved after the incorporation of Ni(OH)2 nanoclusters. The presence of Ni(OH)2 nanoclusters would provide additional water dissociation sites while MoS2 is ready for the adsorption and combination of the generated H*, and this so-called synergistic effect eventually induces significantly enhanced alkaline HER kinetics. Besides, the electron transfer from Ni(OH)2 to MoS2 increases the proton affinity of MoS2. The present results describe an interesting case of an atomic-scale electrochemically inert material promoted HER process, and would open a new avenue into designing efficient hetero-nanostructures towards electrocatalytic applications.


 Please wait while we load your content...
Please wait while we load your content...