Effects of nanobubbles on peptide self-assembly
Abstract
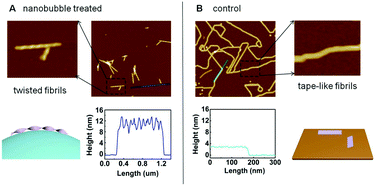
It is believed that the aggregation of amyloid proteins or peptides is promoted by the presence of an air–water interface, and substantial evidence suggests that the characteristics of the air–water interface play critical roles in foam-induced protein aggregation during foam fractionation. However, the effects of the air–water interface on the self-assembly of amyloid-like peptides have not yet been elucidated clearly at the nanometer scale. In this work, air nanobubbles produced in water solution were employed for studying interfacial effects on the self-assembly of a model amyloid peptide termed P11. An atomic force microscopy study showed that the air nanobubbles induced the formation of peptide fibrils with a 9–13 nm helix structure in the P11 solution. Thioflavin T fluorescence and circular dichroism spectroscopic analysis indicated that the nanobubbles induced the change of the peptide conformation to a β-sheet structure. Based on these observations, we have proposed a mechanism to explain how the nanobubbles affect the self-assembly of the P11 peptide at the nanometer scale. Since air nanobubbles are present in water solutions in addition to an air–water interface in normal experiments in vitro, our results indicate that nanobubbles must be taken into account to achieve a complete understanding of protein aggregation events.


 Please wait while we load your content...
Please wait while we load your content...