Charge transfer interactions of pyrazine with Ag12 clusters towards precise SERS chemical mechanism†
Abstract
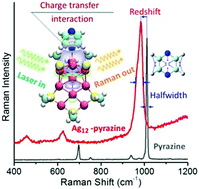
We have synthesized Ag12 nanoclusters (NCs) with mercaptosuccinic acid (H2SMA) as the ligand. This cluster is found to be water-soluble and has satisfactory stability with [Ag12(HSMA)6Na6]2+, as determined by high-resolution mass spectrometry. Interestingly, it is noted that both the H2SMA ligand and Ag12 clusters do not display interference Raman signals, suggesting that this material is a good candidate as a substrate for surface-enhanced Raman spectroscopy (SERS). As a result, we observe enhanced Raman activity of pyrazine molecules adsorbed on Ag12 NCs along with a large red-shift up to ∼27 cm−1. To fully demonstrate the charge transfer interactions between pyrazine and Ag12 clusters, by utilizing first-principles calculations, we estimate polarizability tensor and conduct electronic natural population analysis (NPA), natural bond orbital (NBO) analysis, deformation density analysis (DDA) and charge decomposition analysis (CDA). In view of the minimized contribution from local surface plasmon resonance (LSPR), such a comprehensive study of metal NCs, which are free of Raman interference, provides a modelling method towards the long-debated chemical mechanism in SERS theory.


 Please wait while we load your content...
Please wait while we load your content...