A coupled effect of dehydration and electrostatic interactions on selective ion transport through charged nanochannels†
Abstract
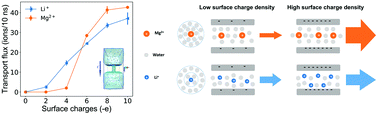
Selective ion transport is an essential feature of biological ion channels. Due to the subnanometer size and negatively charged surface of ion channels, the ion selectivity is affected by both dehydration effects and electrostatic interactions. Their coupled effect on selective ion transport, however, has been elusive. Here, using molecular dynamics simulations, we study ion (Li+ and Mg2+) transport through subnanometer carbon nanotubes (CNTs) with varying charge densities. Our results indicate that the dehydration effect governs the ionic transport at low surface charge densities, hence the nanochannel shows a selectivity for Li+ ions. In contrast, the nanochannel switches to a selectivity for Mg2+ ions as the electrostatic interaction between the cations and the negatively charged wall dominates the transport at high surface charge densities.


 Please wait while we load your content...
Please wait while we load your content...