Targeting myeloid regulators by paclitaxel-loaded enzymatically degradable nanocups†
Abstract
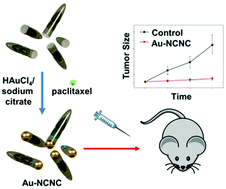
Tumor microenvironment is characterized by immunosuppressive mechanisms associated with the accumulation of immune regulatory cells – myeloid-derived suppressor cells (MDSC). Therapeutic depletion of MDSC has been associated with inhibition of tumor growth and therefore represents an attractive approach to cancer immunotherapy. MDSC in cancer are characterized by enhanced enzymatic capacity to generate reactive oxygen and nitrogen species (RONS) which have been shown to effectively degrade carbonaceous materials. We prepared enzymatically openable nitrogen-doped carbon nanotube cups (NCNC) corked with gold nanoparticles and loaded with paclitaxel as a therapeutic cargo. Loading and release of paclitaxel was confirmed through electron microscopy, Raman spectroscopy and LC-MS analysis. Under the assumption that RONS generated by MDSCs can be utilized as a dual targeting and oxidative degradation mechanism for NCNC, here we report that systemic administration of paclitaxel loaded NCNC delivers paclitaxel to circulating and lymphoid tissue MDSC resulting in the inhibition of growth of tumors (B16 melanoma cells inoculated into C57BL/6 mice) in vivo. Tumor growth inhibition was associated with decreased MDSC accumulation quantified by flow cytometry that correlated with bio-distribution of gold-corked NCNC resolved by ICP-MS detection of residual gold in mouse tissue. Thus, we developed a novel immunotherapeutic approach based on unique nanodelivery vehicles, which can be loaded with therapeutic agents that are released specifically in MDSC via NCNC selective enzymatic “opening” affecting change in the tumor microenvironment.


 Please wait while we load your content...
Please wait while we load your content...