The role of mechanical force on the kinetics and dynamics of electrochemical redox reactions on graphene†
Abstract
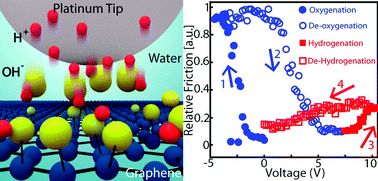
Electrochemical reactions are a critical class of processes strongly influenced by atomic scale effects, where the relationships between local chemical composition, stress, strain, and reactivity are not well understood. Here we investigate the relationship between applied stress and reaction rates for the oxygen evolution reaction on multi-layered graphene using conductive atomic force microscopy. During the reaction, oxygen groups accumulate on the surface and the oxygenation rate increases with applied load. The results also show that the rate is not uniform across the surface, where local edges and defects are more reactive than the basal plane. The results presented here are interpreted in the context of transition state theory, where applied load over the reaction coordinate linearly modifies the energy landscape. This work motivates the general efficacy of atomic force microscopy as a tool to study relationships between local mechanical surface effects and electrochemical reactivity.


 Please wait while we load your content...
Please wait while we load your content...