The role of Li+ in the upconversion emission enhancement of (YYbEr)2O3 nanoparticles†
Abstract
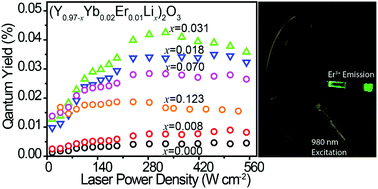
The mechanism of upconversion enhancement for Li+-doped materials is still contentious. Attempting to settle the debate, here the upconversion emission enhancement of (Y0.97−xYb0.02Er0.01Lix)2O3, x = 0.000–0.123, nanoparticles is studied. Li+ incorporation in the Y2O3 host lattice is achieved via co-precipitation and solid-state reaction routes. In contrast to numerous reports, elemental analysis reveals that the former method does not afford Li+-bearing nanoparticles. The solid-state reaction route accomplishes an effective Li+ doping, as witnessed by inductively coupled plasma atomic emission spectroscopy and X-ray photoelectron spectroscopy (XPS). Transmission electron microscopy and powder X-ray diffraction showed an increase in nanoparticle size with increasing Li+ concentration. Rietveld refinement of powder X-ray diffraction data shows that the cubic lattice parameter decreases with increasing Li+ content. The emission quantum yield increases tenfold with increasing Li+ content up to x = 0.123, reaching a maximal value of 0.04% at x = 0.031. XPS and infrared spectroscopy show that the carbonate groups increase with increasing Li+ content, thus not supporting the prevailing view that the upconversion luminescence enhancement observed upon Li+ nanoparticle's doping is due to the decrease of the number of quenching carbonate groups present. Rather, the particle size increment and the decrease in the lattice parameter of the host crystals are shown to be the prime sources of quantum yield enhancement.


 Please wait while we load your content...
Please wait while we load your content...