Phase-selective synthesis of self-supported RuP films for efficient hydrogen evolution electrocatalysis in alkaline media†
Abstract
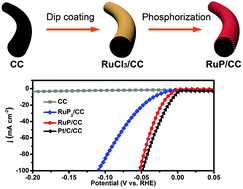
Large-scale hydrogen production through alkaline water electrolysis requires highly active and less expensive materials to replace platinum as a catalyst for the hydrogen evolution reaction (HER). Ruthenium, as the cheapest platinum-group element, has recently been demonstrated to exhibit excellent activity toward the HER. However, achieving better HER activity of ruthenium-based materials by selecting a more active phase is still unexplored. Herein, we report the fabrication of self-supported RuP and RuP2 catalyst films on carbon cloth (RuP/CC and RuP2/CC) via a facile, potentially scalable, and phase-controllable synthetic method. RuP/CC displays superior catalytic activity with a low overpotential of 13 mV at 10 mA cm−1, outperforming RuP2/CC (33 mV at 10 mA cm−1) and most non-Pt HER catalysts. Moreover, good electrochemical stability and faradaic efficiency of nearly 100% are also demonstrated for RuP/CC. Density functional theory calculations reveal that RuP with a higher charge density at the Ru site is more favorable for the chemisorption of hydrogen, thereby exhibiting better HER activity than RuP2.


 Please wait while we load your content...
Please wait while we load your content...