Electrostatically regulated ternary-doped carbon foams with exposed active sites as metal-free oxygen reduction electrocatalysts†
Abstract
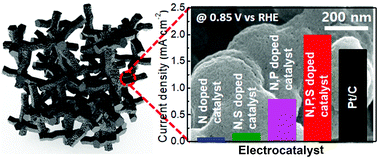
Pt, a representative electrocatalyst for the oxygen reduction reaction (ORR), has suffered from high cost and poor stability, and thus it is essential to develop alternative electrocatalyst with a high catalytic activity comparable to Pt. Herein, we propose a rationally designed metal-free electrocatalyst with exposed active sites using an N, P, and S ternary-doped and graphene-incorporated porous carbon foam. We developed a novel template-free synthetic approach wherein the electrostatically-mediated complexation of graphene oxide (GO) with 2-aminothiazole (2AT) and branched polyethylenimine (PEI) in the presence of phytic acid (PA) was first induced, followed by a carbonization process to drive the formation of a three-dimensionally interconnected porous carbon foam. The resulting electrocatalyst exhibited a high pore volume and greatly extended specific surface area along with exposed active sites. Benefiting from these properties, the synthesized ternary-doped carbon foam displayed an outstanding electrocatalytic activity for the oxygen reduction ORR through four-electron transfer pathways. We observed that the remarkably improved ORR performance of the synthesized materials manifested an onset and a half-wave potential, mostly close to those of the commercially available ORR electrocatalyst of 20 wt% Pt/C while securing a greater stability in alkaline media.


 Please wait while we load your content...
Please wait while we load your content...