Growth of boron nitride nanotubes from magnesium diboride catalysts†
Abstract
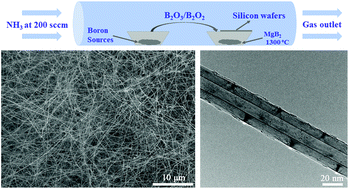
The difficulty in synthesizing boron nitride nanotubes (BNNTs) in a conventional horizontal tube furnace by chemical vapor deposition (CVD) may be ascribed to the failure to identify suitable catalysts and nucleation particles. This report demonstrates that magnesium diboride (MgB2) can effectively catalyze the growth of BNNTs in such a tube furnace from various boron sources, including boron oxide (B2O3), boric acid (H3BO3), and a mixture of boron (B) and calcium oxide (CaO). This catalyst is more efficient than the possible magnesium oxide (MgO) or magnesium nitride (Mg3N2) catalysts. MgB2 efficiently catalyzes the formation of BNNTs by maintaining a liquid state and showing a dissolving capacity for B2O3 at the growth temperature, thus satisfying the criteria for the vapor–liquid–solid (VLS) mechanisms of one-dimensional nanomaterials. First-principles simulations demonstrate that B2O3 can be dissolved into the MgB2 nanoparticle. We believe that the strong catalytic behavior of MgB2 can be attributed to its robust nucleation for BNNTs and dissolubility for B2O3.


 Please wait while we load your content...
Please wait while we load your content...