Visible light driven water splitting through an innovative Cu-treated-δ-MnO2 nanostructure: probing enhanced activity and mechanistic insights†
Abstract
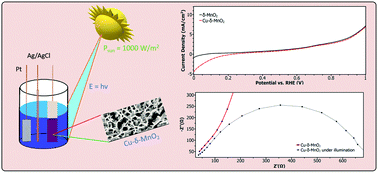
In this study, we have fabricated nanostructured thin films of δ-MnO2 on FTO glass substrates by a facile, room-temperature and low cost chemical bath deposition method. A copper treatment procedure in the synthesis steps results in a film of Cu-δ-MnO2, which displays significant photoactivity when used as a photocathode for hydrogen evolution reaction, with a photocurrent of 3.59 mA cm−2 (at 0 V vs. RHE) in a mild acidic solution. Furthermore, the electrodes also display significant electrocatalytic activity towards water oxidation reaching up to 10 mA cm−2 (at only 1.67 V vs. RHE). The Cu-δ-MnO2 film has been thoroughly characterized via various physicochemical, optical and electrochemical techniques, and an attempt has been made to explain the conductivity mechanism. It is suggested that Cu treatment enhances the photoactivity of δ-MnO2 films through a series of surface dominated processes, which facilitate reduced recombination and enhanced hole consumption at the interface of the electrode and electrolyte. These results establish birnessite-based manganese dioxides as suitable candidates for electrodes in water splitting cells and pave the way for atomic-level engineering of earth abundant materials to reach the ultimate goal of low-cost, sustainable generation of hydrogen.


 Please wait while we load your content...
Please wait while we load your content...