Efficient and simplified nanomechanical analysis of intrinsically disordered proteins†
Abstract
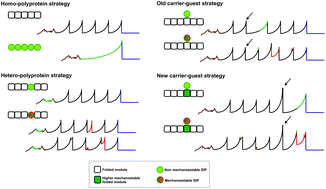
Intrinsically disordered proteins (IDPs) lack a tertiary structure. Amyloidogenic IDPs (aIDPs) in particular have attracted great interest due to their implication in several devastating diseases as well as in critical biological functions. However, the conformational changes that trigger amyloid formation in aIDPs are largely unknown. aIDPs’ conformational polymorphism at the monomer level encumbers their study using bulk techniques. Single-molecule techniques like atomic force microscopy-based single-molecule force spectroscopy represent a promising approach and a “carrier–guest” strategy, in which the protein of interest is mechanically protected, was developed to overcome the spurious signals from the noisy proximal region. However, since the carrier and single-molecule markers have similar mechanostabilities, their signals can intermingle in the force–extension recordings, making peak selection and analysis very laborious, cumbersome and prone to error for the non-expert. Here we have developed a new carrier, the c8C module from the CipC scaffoldin, with a higher mechanostability so that the signals from the protected protein will appear at the end of the recordings. This assures an accurate, more efficient and expert-independent analysis, simplifying both the selection and analysis of the single-molecule data. Furthermore, this modular design can be integrated into any SMFS polyprotein-based vector, thus constituting a useful utensil in the growing toolbox of protein nanomechanics.


 Please wait while we load your content...
Please wait while we load your content...