Long circulatory liposomal maduramicin inhibits the growth of Plasmodium falciparum blood stages in culture and cures murine models of experimental malaria†‡
Abstract
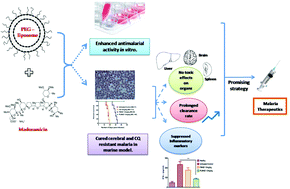
Malaria continues to be one of the deadliest infectious diseases and a global health menace. The emergence and spread of drug-resistant strains of malaria parasites have further made the process of disease management grimmer. Thus, there is an urgent need to identify promising antimalarial strategies that can target the blood stages as well as block parasite transmission. Maduramicin is one such ionophore selected out of a recent screen of gametocytocidal compounds that exhibit potent antiplasmodial activity. However, maduramicin's strong hydrophobic nature and associated toxicity restrict its application in chemotherapy. To alleviate this problem, we have developed a liposomal formulation loaded with the ionophore maduramicin for the treatment of chloroquine sensitive and resistant Plasmodium infections. Here, we show that maduramicin in PEGylated liposomal formulations displayed enhanced antiplasmodial activity in vitro compared to free maduramicin. Significantly, four consecutive doses of 1.5 mg kg−1 body weight of PEGylated maduramicin loaded lipid vesicles completely cured cerebral and chloroquine resistant murine models of malaria without any obvious toxic effects and suppressed the key inflammatory markers associated with the progression of the disease. PEGylated liposomal maduramicin also exhibited a prolonged plasma clearance rate, implying a greater chance of interaction and uptake by infected RBCs. Furthermore, we also provide evidence that the detrimental effect of liposomal maduramicin on parasite survival is mediated by increased ROS generation and subsequent perturbation of parasite mitochondrial membrane potential. This study presents the first report to demonstrate the potent antimalarial efficacy of maduramicin liposomes, a strategy that holds promise for the development of successful therapeutic intervention against malaria in humans.


 Please wait while we load your content...
Please wait while we load your content...