Ferromagnetic Cr2Te3 nanorods with ultrahigh coercivity
Abstract
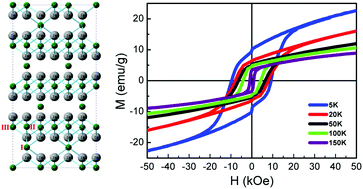
Ferromagnetic Cr2Te3 nanorods were synthesized by a one-pot high-temperature organic-solution-phase method. The crystalline phases and magnetic properties can be systematically tuned by varying the molar ratio of the Cr and Te precursors. A magnetically hard phase, identified as chemically ordered Cr2Te3, is the dominating one at the precursor ratio between Cr : Te = 1 : 1.2 and 1 : 1.8. A magnetically soft phase, attributed to chemical disorder due to composition inhomogeneity and stacking faults, is present under either Cr-rich or Te-rich synthesis conditions. A large coercivity of 9.6 kOe is obtained for a Cr : Te precursor ratio of 1 : 1.8, which is attributed to the large magnetocrystalline anisotropy of ordered Cr2Te3 nanorods, and verified by density-functional theory calculations. The hard and soft phases sharing coherent interfaces co-exist in a seemingly single-crystalline nanorod, showing an unusual transition from exchange-coupled behavior at higher temperatures to two-phase behavior as the temperature is lowered.


 Please wait while we load your content...
Please wait while we load your content...