Heparin assisted assembly of somatostatin amyloid nanofibrils results in disordered precipitates by hindrance of protofilaments interactions†
Abstract
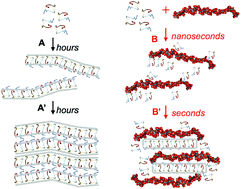
Amyloid nanofibrils are β-sheet rich protein or peptide assemblies that have pathological roles in over 20 neurodegenerative diseases, but also can have essential physiological roles. This wide variety of functions is likely to be due to subtle differences in amyloid structure and assembly mechanisms. Glycosaminoglycans (GAGs), like heparin, are frequently used in vitro to increase the kinetics of assembly of amyloid fibrils. However, little is known about the effects of adding large polymeric sugars on assembly mechanisms and amyloid nanostructures. Here, we provide insights into the kinetics, assembly mechanisms and structural effects of heparin on the self-assembly of a functional-amyloid forming neuropeptide hormone, somatostatin-14. We show that pure somatostatin-14 self-assembles into amyloid fibrils via the formation of antiparallel β-sheet networks, in a typical amyloid aggregation process. These fibrils then laterally assemble into ordered liquid crystalline structures through the generation of further parallel β-sheet networks. If heparin molecules are present, they intercalate between the peptide assemblies during the initial stages of aggregation. This intercalation screens electrostatic repulsions hindering the lateral association of protofilaments, preventing liquid crystal formation and resulting in the rapid formation of disordered micron scale precipitates. Our results show that aggregation promotors like heparin can have large effects not just on the kinetics of aggregation but also on assembly mechanisms, and the architecture of amyloid assemblies. Thus highlighting the dangers of using such polymeric sugars in fundamental studies of amyloid aggregation, especially when drawing conclusions on structure-function relationships or when investigating amyloid-based nanostructures as bionanomaterials.

 Please wait while we load your content...
Please wait while we load your content...