In situ insight into the unconventional ruthenium catalyzed growth of carbon nanostructures†
Abstract
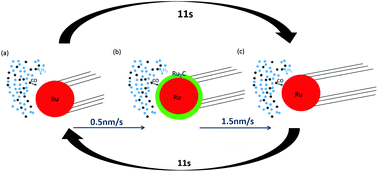
We report on the in situ analysis of the growth process of carbon nanostructures catalyzed by Ru nanoparticles using syngas, a mixture of hydrogen and CO, as the carbon source at a medium temperature (500 °C). The structural modifications of the dual nanotube/nanoparticle system and the general dynamics of the involved processes have been directly followed during the growth, in real time and at the atomic scale, by transmission electron microscopy in an environmental gas cell at atmospheric pressure. After a reduction step under hydrogen and syngas, the particles became very active for the carbon growth. The growth rate is independent of the particle size which mainly influences the nanotube wall thickness. Other subtle information on the general behavior of the system has been obtained, as for instance the fact that the regular changes in the direction of the particle originate generally from the particle shape fluctuation. The main result is the evidence of a new growth mode in relation to the presence and the high instability of the ruthenium carbide phase which acts as a carbon reservoir. For the first time, a relaxation oscillation of the growth rate has been observed and correlated with the metal–carbide structural transition at the particle sub-surface.


 Please wait while we load your content...
Please wait while we load your content...