Near ultra-violet to mid-visible band gap tuning of mixed cation RbxCs1−xPbX3 (X = Cl or Br) perovskite nanoparticles†
Abstract
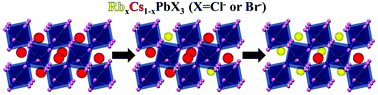
One of the most attractive features of perovskite materials is their chemical flexibility. Due to innovative chemical compositions of perovskites, their optical and structural properties, and functionalities have become more advanced, enabling better solar performance in photovoltaics, as well as robustness and excellent properties in the nanoscale for optoelectronics. The quest for novel perovskite compositions in the nano-scale is significantly important. This paper reports on a mixed-cation system of RbxCs1−xPbX3 (where X = Cl or Br) nanoparticles. The absorption of the nanoparticles is tunable in the near ultra-violet and visible regions between ∼395–525 nm for RbxCs1−xPbX3 (x = 0 to x = 0.8 and X = Cl or Br). The photoluminescence quantum yields (PLQY) of the mixed Rb+/Cs+ nanoparticle systems are comparable to the PLQY of CsPbX3 nanoparticles. Interestingly an attempt to synthesize Cl- and Br-based nanoparticles with high Rb+ content was successful, although possessing low tolerance factors. We conclude that these mixed Rb+/Cs+ nanoparticles are more adjustable to structural distortions caused by cation substitutions than their bulk counterparts, which opens a way towards the development of more advanced mixed-ion perovskite compositions in the nano-scale.


 Please wait while we load your content...
Please wait while we load your content...