Platinum-catalyzed selective N-allylation of 2,3-disubstituted indoles with allylic acetates in water†
Abstract
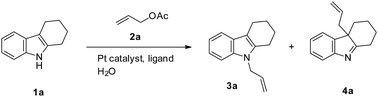
Due to their biological activity, indoles and substituted indoles have attracted considerable attention from both synthetic and medicinal scientists. Much effort has been directed toward the development of methods for the functionalization of the indole nucleus. The protocol uses a catalytic amount of catalyzed platinum as a promoting agent, producing N-allylated indoles in considerable yields. Moreover, water, with its large heat capacity, is one of the most abundant molecules on earth. The use of water as a solvent may bring about many environmental benefits. Herein, we have demonstrated that the platinum-catalyzed selective N-allylation of 2,3-disubstituted indoles proceeds in water. This method provides a simple, convenient, and efficient way to afford a high yield of N-allylated indoles.


 Please wait while we load your content...
Please wait while we load your content...