Facile synthesis of 1,4-benzodiazepine-2,5-diones and quinazolinones from amino acids as anti-tubercular agents†
Abstract
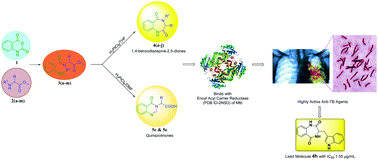
A family of 1,4-benzodiazepine-2,5-diones and quinazolinones with diverse substituents at the C-3 position were synthesized via a novel, simple and convenient methodology using H2PtCl6 as the catalyst. The substitution at the C-3 position was varied using pre-defined amino acids as precursors. The synthesized benzodiazepines were screened for anti-mycobacterial tuberculosis (anti-TB) activity. The results revealed that the 1,4-benzodiazepine-2,5-diones displayed promising activity in comparison with their open chain precursors, which indicates that the diazepine frame is vital for their activity. The compounds 4h and 4f were found to be the lead nominees in the series with MIC values of 1.55 and 2.87 μg mL−1, respectively. A docking study was carried out on the enoyl acyl carrier protein to provide a better understanding of the mechanism of action of these compounds. Based on this study, the 1,4-benzodiazepine-2,5-dione framework is a good starting point for the development of new lead drug candidates in the treatment of multi-drug resistant tuberculosis.


 Please wait while we load your content...
Please wait while we load your content...