One-pot synthesis of novel functionalized benzodiazepines via three-component or domino reactions†
Abstract
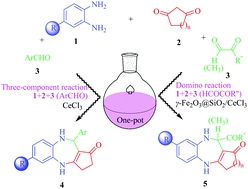
Several novel three-component or domino reactions that enable a variety of unique heterocyclic scaffolds through carbon–carbon and carbon–nitrogen bond formations are herein reported. These reactions employing a one-pot strategy provide an efficient approach for the synthesis of benzodiazepines with tricyclic or tetracyclic systems containing aryl, carboxyl, ester and acyl groups. The main objective of this methodology is to achieve the compatibility of various functional groups via introducing active groups under mild reaction conditions. 40 new compounds were successfully synthesized in good to excellent yields (82–97%) from readily available 1,2-phenylenediamine, β-cyclodione and aldehyde derivatives by using a mild catalyst (γ-Fe2O3@SiO2/CeCl3). Structures of all the products were fully confirmed by spectroscopic techniques and by recording the single crystal XRD of 5bac. Moreover, a plausible reaction mechanism has been proposed.


 Please wait while we load your content...
Please wait while we load your content...