Synthesis of TiO2/rGO composites with different morphologies and their electrocatalysis for the oxygen reduction reaction†
Abstract
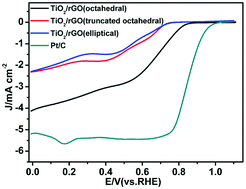
The replacement of platinum (Pt) with nonprecious metals and their oxides that possess superior catalytic activity and stability toward the oxygen reduction reaction (ORR) remains challenging for the development of fuel cell devices. Herein, reduced graphene oxide (rGO)-supported (TiO2) composites with different morphologies (octahedral, truncated octahedral and elliptical) are successfully synthesized through a hydrothermal strategy. Among the TiO2/rGO composites with different morphologies, the composite containing octahedral TiO2 (called TiO2/rGO (octahedral)) exhibits the best ORR catalytic activity in alkaline solution, with the half-wave potential being almost equal to that of commercial Pt/C with four electrons involved in the transfer. Most importantly, this composite displays a much better long-term stability and methanol tolerance than Pt/C. The relationship between the morphology and the catalytic activity of TiO2/rGO as a cathode catalyst was further studied through Arrhenius equation calculations. This reveals that TiO2/rGO (octahedral) has a lower apparent activation energy, which is beneficial for increasing the rate of electron transfer and oxygen adsorption during the ORR. We believe that our findings provide a new method for the rational design of an inexpensive and highly active ORR catalyst.


 Please wait while we load your content...
Please wait while we load your content...