8-Amino-5,6,7,8-tetrahydroquinoline in iridium(iii) biotinylated Cp* complex as artificial imine reductase†
Abstract
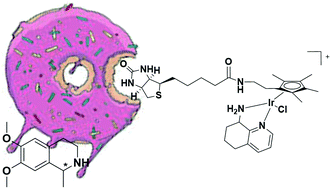
Diamine ligands I–IV coordinated to an iridium metal complex with the biotin moiety anchored to the Cp* ring were investigated. This strategy, in contrast to the traditional biotin–streptavidin technology that uses a biotinylated ligand in the artificial imine reductase, is practical for envisaging how the enantiodiscrimination by different Streptavidin (Sav) mutants could influence the chiral environment of the metal cofactor. Only in the case of (R)-CAMPY IV did the chirality at the metal centre and the second coordination sphere environment, which was dictated by the host protein, operate in a synergistic way, producing better enantioselectivity at a S112M Sav catalyst/catalyst ratio of 1.0 : 2.5. Under these optimized conditions, the artificial imine reductase afforded a good enantiomeric excess (83%) in the asymmetric transfer hydrogenation of 6,7-dimethoxy-1-methyl-3,4-dihydroisoquinoline.


 Please wait while we load your content...
Please wait while we load your content...