Green synthesis of porous Au–Nx-TiO2 nanospheres for solar light induced photocatalytic degradation of diazo and triazo dyes and their eco-toxic effects†
Abstract
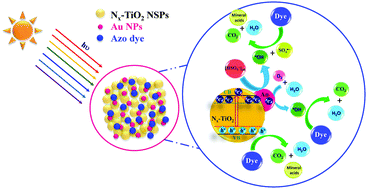
Porous TiO2 nanospheres and nitrogen doped TiO2 nanospheres (Nx-TiO2 NSPs) in the range of 150 to 200 nm were synthesized through a facile one pot green synthesis route and their surface was modified using plasmonic gold nanoparticles (Au NPs) without altering their anatase phase. These nanospheres were characterized using X-ray diffraction (XRD), X-ray photoelectron spectra (XPS), Brunauer–Emmett–Teller (BET) surface area measurements, and scanning electron microscope (SEM) and transmission electron microscope (TEM) analyses. The gold deposited photocatalysts (porous Au–Nx-TiO2 and Au–TiO2 NSPs), by virtue of their visible-light absorption properties and the rapid charge separation at the Au/TiO2 and Au/Nx-TiO2 interfaces, demonstrated an excellent photocatalytic activity under direct solar light irradiation towards the degradation of diazo and triazo dyes such as reactive red 120 (RR120) and direct blue 71 (DB71), respectively. The correlation between the amount of HO˙ formation and the amount of photocatalytic degradation indicated that the photocatalytic degradation of the azo dyes increased with the respective HO˙ formation. In addition, the real time applications of the photocatalytic degradation of both diazo and triazo dyes were studied in tap water. The eco-toxicity study disclosed that the toxicity of the degradation intermediates was effectively removed with respect to the irradiation time owing to the mineralization of both azo dyes and their intermediates. The stability study revealed the photocatalytic activity of Au–Nx-TiO2 NSPs in upto eight consecutive cycles without any reduction of their initial photocatalytic activity. The results indicated that industrial wastewater in large volumes can be processed rapidly using the porous Au–Nx-TiO2 NSPs just by exploiting sunlight.


 Please wait while we load your content...
Please wait while we load your content...