Iron(iii) identification and proton conduction of a luminescent cadmium–organic framework†
Abstract
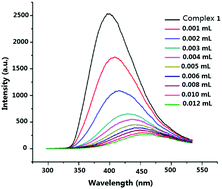
The multifunctional organic bridging ligand, 2-phenyl(3,4-dimethyl)-1-H-imidazole-4,5-dicarboxylic acid (H3DMPhIDC), was reacted with Cd(II) ions to form a luminescent metal–organic framework, [Cd(HDMPhIDC)(H2O)]n (1), which has been successfully synthesized and structurally characterized. MOF 1 shows 1D chain structure and good recognition performance for Fe3+ ions. The luminescent quenching efficiency for iron(III) is 89.46% and the detection limit is 3.98 × 10−6 M in aqueous solution. The recognition mechanism of Fe3+ has been explored by UV-Vis and PXRD determinations. Furthermore, the proton conduction of MOF 1 has been investigated. Interestingly, 1 has a high proton conductivity value of 1.30 × 10−4 S cm−1 at 100 °C and 98% RH. The large activation energy indicates that proton transfer in 1 through the vehicular mechanism.


 Please wait while we load your content...
Please wait while we load your content...