A transition metal-free approach towards synthesis of β-carboline tethered 1,3,4-oxadiazoles via oxidative C–O bond formation†
Abstract
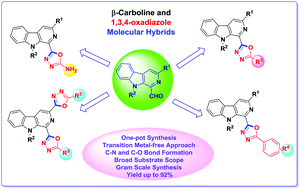
An efficient protocol has been developed for one-pot synthesis of biologically interesting β-carboline substituted 1,3,4-oxadiazoles via an I2-assisted oxidative C–O bond formation strategy. This metal-free sequential approach is found to be compatible with diversely substituted 1-formyl β-carbolines and aromatic as well as aliphatic hydrazides, providing access to a variety of multifunctional β-carboline linked 1,3,4-oxadiazole derivatives in good to excellent yields. The methodology was found to be applicable to gram scale synthesis of β-carboline substituted 1,3,4-oxadiazole derivatives. Additionally, β-carboline C1 linked 2-amino-1,3,4-oxadiazoles and bis-1,3,4-oxadiazoles were also synthesized using the same strategy.


 Please wait while we load your content...
Please wait while we load your content...