Tris functionalized Cu-centered cyclohexamolybdate molecular armor as a bimetallic catalyst for rapid p-nitrophenol hydrogenation†
Abstract
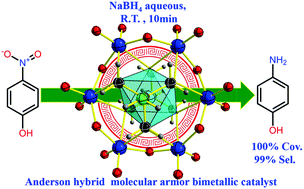
A water-soluble tris functionalized Cu-centered Anderson nanocluster (NH4)4{[NH2C(CH2O)3]2CuMo6O18} with a remote NH2 motif was synthesized for the first time. The bimetallic cluster's structure is identified by single crystal X-ray diffraction, and it is also further well characterized by a combination of technologies, such as XPS, TGA, FT-IR spectroscopy, UV-Vis spectroscopy, ESI-MS, and EA spectroscopy. It can serve as a non-noble metal containing bimetallic catalyst to afford rapid reduction of aqueous p-nitrophenol to p-aminophenol (10 min) with a promising conversion (a decent 100%) and perfect selectivity (ca. 99%) in aqueous solution at room temperature. The catalytic reaction rate constant of the bimetallic {[NH2C(CH2O)3]2CuMo6O18}4− catalyst was one order higher (ca. 9.4 times) than that of the corresponding monometallic [Mo7O24]6− catalyst. The bimetallic cluster shows good catalytic performance and recyclability with an intact structure, confirmed by powder XRD. Cyclic voltammetry investigation indicated that a reversible two one-electron process was observed, in which CuII/CuI and MoVI/MoV were the redox couples and served as an ideal electrochemically stable multi-electron reservoir to promote the reduction process that enabled an obvious bimetallic synergistic catalytic performance during such p-nitrophenol hydrogenation reduction.


 Please wait while we load your content...
Please wait while we load your content...