Synthesis, crystal structures, photoluminescence, magnetic and antioxidant properties, and theoretical analysis of Zn(ii) and Cu(ii) complexes of an aminoalcohol ligand supported by benzoate counter anions†
Abstract
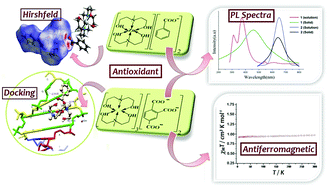
The coordination chemistry of two complexes [Zn(Me-deaH2)2][ba]2 (1) and [Cu(Me-deaH2)]3[tca]2 (2), where Me-deaH2 = N-methyldiethanolamine, Hba = benzoic acid and H3tca = 1,2,4-benzenetricarboxylic acid, has been explored. The Zn(II) and Cu(II) complexes are well characterized using spectral, single crystal X-ray and magnetic studies. The X-ray structure reveals a distorted octahedral geometry around both the metal ions. While the aminoalcoholate is coordinated with the metal ions, the benzoate moeity is present as a counter anion consolidating the crystal structure. Furthermore, the non-covalent interactions C–H⋯π, C–H⋯O, O–H⋯O and C–H⋯C result in 2D sheet networks in 1 and 2. These non-covalent interactions are theoretically predicted by Hirshfeld surface analysis. Solid state photoluminescence (PL) spectra of the complexes confirm their better luminescent behaviour than the corresponding parent conjugated ligands. The temperature variable magnetic data disclose the weak antiferromagnetic behaviour of 2. The plot indicates that the Cu(II) complex follows the Curie–Weiss law with a Curie constant, C = 0.29 cm3 K mol−1 and a Weiss constant, θ = −0.39 K. Moreover, the binding ability of both the complexes with DNA (PDB ID: 1BNA) is investigated theoretically and the data reveal the free energy of binding to be −256.22 (1) and −357.72 kcal mol−1 (2). The higher binding affinity of 2 can be rationalized in terms of its greater extent of H-bonding with DNA. Moreover, the assessment of the antioxidant properties employing DPPH free radical scavenging [IC50 = 0.628 ± 0.005 (1) and 0.850 ± 0.005 mg mL−1 (2)] and hydrogen peroxide assay [IC50 = 0.068 ± 0.001 (1) and 0.76 ± 0.01 mg mL−1 (2)] ascertains the Zn(II) complex to be a potent antioxidant.


 Please wait while we load your content...
Please wait while we load your content...