The first general synthesis of 2-C-(β-d-glycopyranosyl)pyrimidines and their evaluation as inhibitors of some glycoenzymes†
Abstract
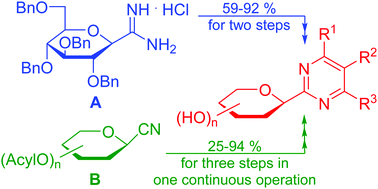
A systematic study was performed on the preparation of unknown 2-C-(β-D-glucopyranosyl)pyrimidines. Pinner type cyclisation of O-perbenzylated C-(β-D-glucopyranosyl)formamidine with β-ketoesters, dimethyl malonate, and β-diketone derived α,β-unsaturated β-chloroketones followed by catalytic hydrogenation resulted in various substituted 2-C-(β-D-glucopyranosyl)-pyrimidin-4(3H)-ones, and 2-C-(β-D-glucopyranosyl)-4,6-disubstituted-pyrimidines, respectively, in moderate to good yields. The above pyrimidine derivatives were also obtained by ring closure of the unprotected C-(β-D-glucopyranosyl)formamidine with the same 1,3-dielectrophiles. In addition, a continuous one-pot three-step procedure starting from O-peracylated D-glycopyranosyl cyanides was also elaborated to give representatives of the aforementioned pyrimidines with various sugar configurations in acceptable to excellent overall yields (25–94%). Due to the versatility of the applied 1,3-dielectrophiles, these synthetic routes represent the first expansible method to obtain the target compounds. The new C-glycopyranosyl pyrimidines showed moderate inhibition against α-glucosidase and β-galactosidase enzymes, had, however, no activity against glycogen phosphorylase. The obtained molecule library is ready for further biological testing.


 Please wait while we load your content...
Please wait while we load your content...