Facile synthesis of novel 3,4,5-trisubstituted-1,2,4-triazin-6(1H)-ones via a sequential Ugi–Smiles type/nucleophilic substitution/cyclization reaction†
Abstract
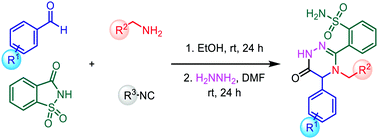
A simple and innovative strategy is described for synthesizing a library of novel 3,4,5-trisubstituted-1,2,4-triazin-6(1H)-one backbones in mild conditions. This approach involves multiple bond-forming events in an Ugi–Smiles type reaction and the post-condensation of the Ugi–Smiles adduct incorporating a series of electron-withdrawing substituted benzaldehydes, primary amines, saccharin, and isocyanides followed by the addition of hydrazine hydrate for the post-condensation step.


 Please wait while we load your content...
Please wait while we load your content...