An aqueous medium-controlled stereospecific oxidative iodination of alkynes: efficient access to (E)-diiodoalkene derivatives†‡
Abstract
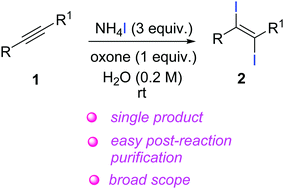
A new and versatile approach for the stereospecific iodination of alkynes using cheap, air stable and non-toxic reagents in aqueous media has been developed. This protocol is tolerant of various functional groups, and provides a broad range of vicinal diiodoalkenes with exceptional E-selectivity under mild conditions. Scale-up reactions (up to 5 g) established the proficiency of this protocol and highlight the feasibility of large scale reactions.


 Please wait while we load your content...
Please wait while we load your content...