Copper-catalyzed regioselective dehydrogenative alkoxylation of morpholinonyl alkenols: application to the synthesis of spirotricyclic dihydropyrans and of trans-fused bicyclic morpholines†
Abstract
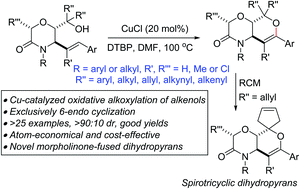
A base-free, regioselective and atom-economical approach to aryl-substituted as well as morpholine-fused dihydropyrans, by Cu-catalyzed intramolecular dehydrogenative alkoxylation of allylic morpholinols, is described. Spirotricyclic dihydropyrans are affordable after ring-closing metathesis.


 Please wait while we load your content...
Please wait while we load your content...