Packing polymorphism in 3-amino-2-pyrazinecarboxylate based tin(ii) complexes and their catalytic activity towards cyanosilylation of aldehydes†
Abstract
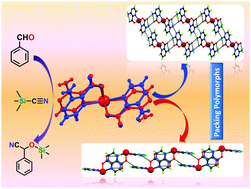
Reactions of 3-amino-2-pyrazinecarboxylic acid (HL) with SnCl2 under different hydrothermal conditions and crystallization processes produced two new mononuclear Sn(II) compounds (1 and 2) of general formula [Sn(κN,O-L)2] (L = 3-amino-2-pyrazinecarboxylate), and single crystal X-ray diffraction analysis indicated that they are packing polymorphs, complex 1 crystallizing in the monoclinic C2/c space group whereas 2 in the monoclinic P21/c. The Hirshfeld surface analysis shows that the supramolecular features of both forms are guided by Sn⋯O and Sn⋯N contacts, as well as by strong N–H⋯O and N–H⋯N hydrogen bonds that differentiate the packing in the two forms. Complex 1 shows a one dimensional hydrogen bonded network whereas 2 shows a 2D hydrogen bonded network. To the best of our knowledge no examples of packing polymorphs based on tin(II) complexes have been reported so far. These polymorphs are interconvertible when immersed in a suitable solvent or mixture of solvents. They also act as heterogeneous catalysts for the cyanosilylation reaction of aldehydes with trimethylsilyl cyanide (TMSCN) and can be recycled several times without losing activity.


 Please wait while we load your content...
Please wait while we load your content...