Designed functionalization of reduced graphene oxide for sorption of Cr(vi) over a wide pH range: a theoretical and experimental perspective
Abstract
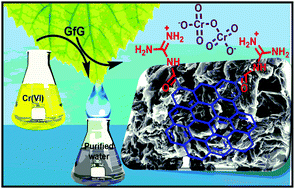
The present study illustrates a clear functionalization chemistry for the synthesis of guanidine functionalized reduced graphene oxide (GfG). GfG has been successfully employed as an effective and selective adsorbent for the removal of noxious Cr(VI) from aqueous pollutants over a wide pH range. Different kinetic models, isotherm studies and thermodynamic parameters have been compared to elucidate the Cr(VI) adsorption chemistry and pathway. The formation of GfG and its active performance towards Cr(VI) remediation is evidenced from microscopic and different spectroscopic analyses. Finally, an attempt has been made by considering DFT based theoretical models to illuminate the mechanism of Cr(VI) amputation by GfG.


 Please wait while we load your content...
Please wait while we load your content...