NIS-Mediated intermolecular hydroamination of allenamides with imidazole heterocycles: a facile protocol for the synthesis of allylic N,N-acetals†
Abstract
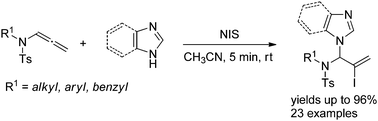
Allylic N,N-acetals are important intermediates in the synthesis of biologically active heterocycles and natural products. Herein, we report a facile protocol for the synthesis of this compound through N-iodosuccinimide-mediated hydroamination of allenamides by imidazole heterocycles. The reaction is regioselective, fast, and tolerant of a broad scope of imidazole and benzimidazole derivatives. The key intermediate is a conjugated sulfimide ion species that undergoes nucleophilic attack by imidazole to form the 1,2-adduct. Mixtures of N1- and N3-substituted isomers were obtained using asymmetrically substituted imidazoles. However, the 1,4-adduct was obtained using a tri-substituted imidazole. The efficiency of the gram-scale reaction suggests the potential industrial application of this synthetic method.


 Please wait while we load your content...
Please wait while we load your content...