Towards a general synthesis of di-aza-amino acids containing peptides†
Abstract
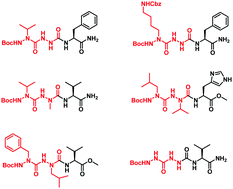
While the incorporation of one aza-amino acid in peptides has been proved to be beneficial for inducing a structure constraint, increasing the resistance towards proteolysis and improving the biological activity, only very rare examples of the incorporation of two or more consecutive aza-amino acids have been reported. In this work, we demonstrate that this fact is probably due to the unsuspected difficulty in synthesizing such peptide analogues, as illustrated by the synthesis of tripeptide derivatives containing two consecutive aza-amino acids. Herein, we report some general guidelines regarding the activation and the coupling of alkyl-hydrazides either mutually or with a natural amino acid, taking into account their nucleophilicity and the nature of their side chains.


 Please wait while we load your content...
Please wait while we load your content...