Two-acceptor one-donor random terpolymers comprising thiophene- and phenyl-capped diketopyrrolopyrrole for organic photovoltaics†
Abstract
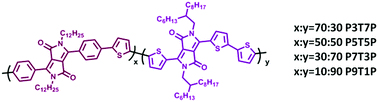
A series of random terpolymers comprising two electron deficient phenyl (PDPP) and thiophene (ThDPP)-capped diketopyrrolopyrrole (DPP) in conjugation with the electron-donating thiophene moiety are synthesised using Stille coupling. Their optical properties, energy levels, hole mobility, crystallinity and solar cell device performance can be systematically fine-tuned by controlling the molar ratio between ThDPP/PDPP (30/70, 50/50, 70/30, and 90/10) contents in the polymer backbone. Herein, we find that the crystalline properties and hole mobility of the terpolymer are enhanced by increasing ThDPP content in the polymer backbone. However, increasing PDPP content leads to low hole mobility and weak crystalline features. These characteristic features afford remarkable effect on the solar cell device performance. Bulk heterojunction (BHJ) solar cells are constructed by using these random terpolymers as donor materials and [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as the acceptor. The best device performances are obtained for polymer P5T5P with the ThDPP/PDPP ratio of 50/50 and power conversion efficiency (PCE) of 2.9% due to balanced charge carrier mobility and optimized crystallinity in addition to good miscibility and favorable surface morphology with the fullerene acceptor. This study demonstrates that improved control of the crystallinity of the polymer donor through structural engineering can greatly help in improving device performance.


 Please wait while we load your content...
Please wait while we load your content...