H-Bonding and C–H⋯π assisted mechanofluorochromism of triphenylamine-containing vinylheterocycles bearing cyano and methyl groups†
Abstract
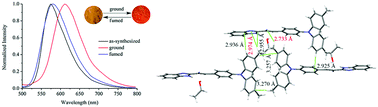
New D–π–A-type triphenylamine-containing benzimidazoles, benzoxazoles and benzothiazoles have been synthesized. It was found that the introduction of a cyano group in vinyl and methyl in triphenylamine was favorable for producing mechanofluorochromism of the fused heterocyclic derivatives. The single crystal X-ray diffraction data revealed that the intermolecular interactions of the H-bonding formed from the cyano group and the C–H⋯π interaction formed from the methyl group would rigidify the molecular conformations, leading to strong solid-state emission. Interestingly, the solid-state emission of the synthesized triphenylamine-containing fused heterocycles bearing cyano and/or methyl groups could be tuned by external mechanical forces on account of the transition between crystalline and amorphous states or between a highly ordered crystalline state and a less ordered state.


 Please wait while we load your content...
Please wait while we load your content...