Antiproliferative activity of new 2-glyco-3-nitro-1,2-dihydroquinolines and quinolines synthesized under solventless conditions promoted by neutral alumina†
Abstract
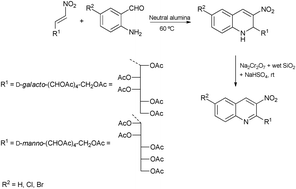
This paper describes the syntheses of new 2-glyco-3-nitro-1,2-dihydroquinolines and 2-glyco-3-nitroquinolines by one-pot aza-Michael–Henry–dehydration reactions using a minimal amount of solvent and neutral alumina as the heterogeneous catalyst. The reactivity of the nitro group–double bond system has also been investigated; thus, the addition of indole or pyrrole to N-formylated 1,2-dihydroquinolines has been studied. Finally, the cytotoxicity and antiproliferative activity of these new compounds have been evaluated against a panel of six human solid tumor cell lines and compared to pharmacological reference compounds, finding that their activity is in the low micromolar range and that the carbohydrate moiety configuration modulates the GI50 values.


 Please wait while we load your content...
Please wait while we load your content...