Lophine and pyrimidine based photoactive molecular hybrids. Synthesis, photophysics, BSA interaction and DFT study†
Abstract
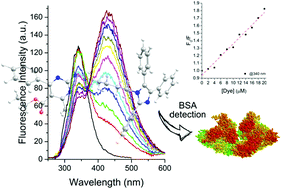
Two photoactive molecular hybrids containing both lophine and pyrimidine moieties were synthesized by multicomponent reaction. The compounds present absorption in the UV-region (below 300 nm) and fluorescence emission in the violet region due to the lophine moiety. Experimental evidence indicates that photoinduced electron transfer (PET) occurs in the excited state of the hybrids. The observed photophysical features were successfully used to explore the interaction of the compounds with bovine serum albumin (BSA) in phosphate buffer solution (PBS) where a significant suppression mechanism was observed for both molecular hybrids. The DFT and TD-DFT calculations were performed at the ωB97XD level of theory. The results show low influence of the solvent on the wavelengths. However, the dipole moment undergoes a significant modification when the solvent is changed, showing a high polar behavior towards the excited state. Calculations also show us that the excitation is local, and that there is no charge transfer.


 Please wait while we load your content...
Please wait while we load your content...