Manganese(ii) complexes with the non-steroidal anti-inflammatory drugs naproxen and mefenamic acid: synthesis, structure, antioxidant capacity, and interaction with albumins and DNA†
Abstract
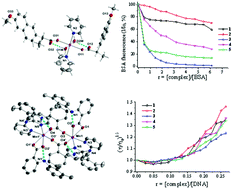
The interaction of the non-steroidal anti-inflammatory drugs naproxen or mefenamic acid with MnCl2 in the presence of the nitrogen-donor ligands 2,2′-bipyridine, 1,10-phenanthroline, 2,2′-bipyridylamine or pyridine resulted in the formation of one dinuclear and four mononuclear Mn(II) complexes. The complexes were characterized by diverse physicochemical and spectroscopic techniques and single-crystal X-ray crystallography. The biological activity of the complexes was evaluated in regard to their ability to scavenge the free radicals of 1,1-diphenyl-2-picrylhydrazyl, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and hydroxyl and their binding affinity towards calf-thymus (CT) DNA and serum albumins. The complexes exhibit noteworthy scavenging activity towards ABTS and hydroxyl radicals; they can bind tightly to CT DNA via intercalation and can bind to bovine or human serum albumins tightly and reversibly.


 Please wait while we load your content...
Please wait while we load your content...