Mesoporous silica supported cobalt catalysts for gas phase hydrogenation of nitrobenzene: role of pore structure on stable catalytic performance†
Abstract
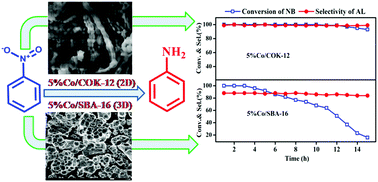
Highly dispersed cobalt nanoparticles were prepared over mesoporous silica with different pore structures (2D-hexagonal COK-12 and 3D-cubic SBA-16). These catalysts were evaluated for gas phase hydrogenation of nitrobenzene to aniline at atmospheric H2 pressure. A combination of catalytic activity and characterization results were assessed to establish the role of the support pore structure on hydrogenation activity. XRD, N2-physisorption, SEM and TEM analysis confirmed the presence of mesoporous structures in the supported cobalt catalysts. H2-TPR, H2-pulse chemisorption and TEM studies demonstrated higher dispersion of cobalt nanoparticles in Co/SBA-16 than in the Co/COK-12 catalyst. During the time-on-stream study the Co/SBA-16 catalyst experienced a gradual deactivation whereas the Co/COK-12 catalyst exhibited constant catalytic performance with respect to the hydrogenation of nitrobenzene. The interconnected cage type pores in Co/SBA-16 catalyst allowed the product molecules to participate in further reactions. This resulted in the formation of condensed products and coke deposition. The Co/SBA-16 catalyst was rapidly deactivated due to pore blocking through coke deposition. N2-Physisorption, TGA, H2-TPR and CHNS elemental analysis of spent catalysts confirmed the severe coke deposition in the Co/SBA-16 catalyst compared to the Co/COK-12 catalyst.


 Please wait while we load your content...
Please wait while we load your content...