ISE-potentiometric sensor for the determination of zolmitriptan: applications in plasma, pharmaceutical formulation and in vitro release profile
Abstract
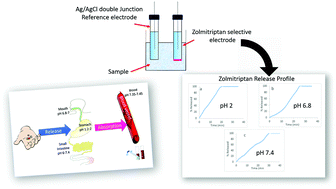
Six different sensors were fabricated and compared for the potentiometric determination of a widely used serotonin receptor agonist zolmitriptan (ZT), which is mainly used for the treatment of acute migraine attacks. Owing to its five-membered lactone ring, ZT was found susceptible to alkaline hydrolysis. The composition of PVC-membrane was systematically optimized via comparing the responses of different membranes. Membranes 1 to 4 contained dioctyl sebacate (DOS) in addition to different ion exchangers, namely, sodium tetraphenylborate (sensor 1), sodium phosphotungstate (sensor 2), sodium phosphomolybdate (sensor 3), and ammonium reineckate (sensor 4). The third sensor demonstrated the best sensitivity with near-Nernstian slope and was selected for the next step. To assess the plasticizer effect, the relatively non-polar plasticizer DOS used in sensor 3 was compared to high dielectric constant plasticizer nitrophenyl octyl ether (NPOE) in sensor 5; it was observed that the latter possessed better sensitivity (LOQ = 6.66 × 10−6 mol L−1), slope (52.821 mV decade−1), and response time. The effect of ionophore on sensor performance was evaluated, where hydroxypropyl β-cyclodextrin was further incorporated as an ionophore (sensor 6), resulting in remarkably higher sensitivity (LOQ = 3.33 × 10−6 mol L−1), better near-Nernstian slope (59.915 mV decade−1), and faster response time. The pH and temperature effects as well as the selectivities of the ionophore-free membrane (sensor 5) and the ionophore-containing membrane (sensor 6) were compared to evaluate the effect of the ionophore on the sensor performance. The developed sensors (5 and 6) were found to be selective for ZT in the presence of different interfering ions as well as a ZT alkaline degradation product. The sensor performances were assessed as per IUPAC recommendations and used for the determination of ZT in the presence of its degradation product in the raw material, orodispersable tablets, and in spiked plasma samples. The stability of sensor response over a wide pH range (pH 2 to 8) enabled selective inline ZT release profile monitoring at different physiological pH values in the presence of its hydrolytic degradation product.


 Please wait while we load your content...
Please wait while we load your content...