Melamine formaldehyde–metal organic gel interpenetrating polymer network derived intrinsic Fe–N-doped porous graphitic carbon electrocatalysts for oxygen reduction reaction†
Abstract
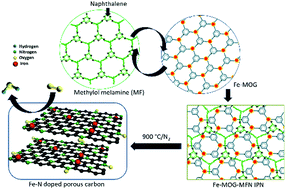
Fe, N doped porous graphitic carbon electrocatalyst (Fe-MOG-MF-C), obtained by pyrolysis of an Interpenetrating Polymer Network (IPN) comprised of melamine formaldehyde (MF as hard segment) and Metal–Organic Gel (MOG as soft segment), exhibited significant Oxygen Reduction Reaction (ORR) activity in alkaline medium. BET surface area analysis of Fe-MOG-MF-C showed high surface area (821 m2 g−1), while TEM, Raman and XPS results confirmed Fe and N co-doping. Furthermore, a modulated porous morphology with a higher degree of surface area (950 m2 g−1) has been accomplished for the system (Fe-MOG-MFN-C) when aided by a sublimable porogen, such as naphthalene. XPS results further demonstrated that these systems exhibited a better degree of distribution of graphitic N and an onset potential value of 0.91 V vs. RHE in 0.1 M KOH solution following an efficient four-electron ORR pathway. The electrocatalytic activity of Fe-MOG-MFN-C is superior to that of Fe-MOG-MF-C by virtue of its higher graphitic N content and surface area. Thus, the study presents a new class of IPN derived MF-MOG nanocomposites with the potential to generate extended versions of in situ Fe–N doped porous graphitic carbon structures with superior ORR activity.


 Please wait while we load your content...
Please wait while we load your content...