Development of recyclable chiral macrocyclic metal complexes for asymmetric aminolysis of epoxides: application for the synthesis of an enantiopure oxazolidine ring†
Abstract
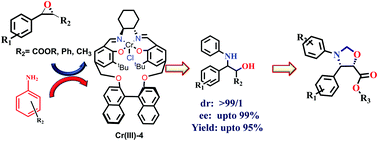
New recyclable chiral salen complexes Cr(III)1–7 were synthesized from various macrocyclic chiral ligands having multistereogenic chiral centers which work synergistically to give the best results in terms of the enantioselectivity and yield of the products. Among the synthesized complexes, the Cr(III)-4 salen complex efficiently catalyzed the ring opening reaction of various aromatic ester epoxides, trans-epoxides and meso-epoxides with anilines to furnish the corresponding β-amino-α-hydroxyl esters and β-amino alcohols with an excellent ee of up to 99% and a high yield of up to 95%. Furthermore, the chirally pure β-amino-α-hydroxyl esters were converted into pharmaceutically important oxazolidine ring systems. Moreover, the chromium catalyst was recycled up to 5 cycles with the retention of its activity.


 Please wait while we load your content...
Please wait while we load your content...