Coumarin-substituted 1,2,4-triazole-derived silver(i) and gold(i) complexes: synthesis, characterization and anticancer studies†
Abstract
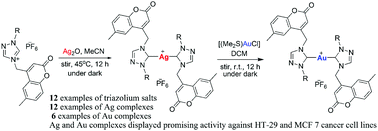
Metal-based small molecules are extensively studied for their potential application in cancer treatment. In this regard, a series of sterically-tuned coumarin-substituted 1,2,4-triazole-based NHC (N-heterocyclic carbene) precursors (1–12) and their silver(I) complexes of the type [NHC–Ag–NHC]+PF6− (13–18) and [NHC–Ag–OAc] (OAc: acetate) (19–24), and gold(I)–NHC (25–30) complexes have been synthesized and thoroughly characterized. The molecular structures of NHC precursors 7, 9 and 10, and silver(I) NHC complexes 13, 14, 15 and 16 have been unambiguously established by the single crystal X-ray diffraction method. The silver centers have a distorted linear coordination geometry, and there is an inversion center at the metal atom with a series of anagostic-like interactions between the silver atom and different CH modules of NHC ligands. Further, the in vitro anticancer activities of the silver(I) (13–18) and gold(I) (25–30) complexes toward two human-derived cancer cell lines, the breast cancer (MCF 7) and the colon cancer (HT-29) cell lines have been examined by SRB assay. All the silver(I) complexes displayed higher anticancer potentials than their gold(I) counterparts toward both cell lines tested, except for complex 18 against MCF 7 cells. Notably, complex 17 exhibited the highest anticancer potency as compared to other members of the series with the GI50 value of 0.3540 ± 0.032 and 8.5983 ± 0.98 μM against the MCF 7 and HT-29 cell lines, respectively.


 Please wait while we load your content...
Please wait while we load your content...