Bio-activated intramolecular anti-aza-Michael addition: stereoselective synthesis of hydantoin derivatives†
Abstract
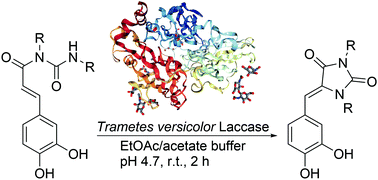
In this paper, we report for the first time a stereoselective synthesis of biologically remarkable hydantoins through an intramolecular anti-aza-Michael addition, activated by the Trametes versicolor Laccase enzyme. This simple and straightforward synthesis affords the products with good yields. A study of the reaction mechanism has been carried out based on DFT calculations, showing that the only possible pathway at room temperature is the anti-aza-Michael addition, due to the very low free energy of activation barrier. We also show that the selective formation of the more stable Z-diastereomer is due to the greater stability of the appropriate conformer during the re-aromatization of the system.


 Please wait while we load your content...
Please wait while we load your content...