Catalytic decomposition of undiluted methane into hydrogen and carbon nanotubes over Pt promoted Ni/CeO2 catalysts†
Abstract
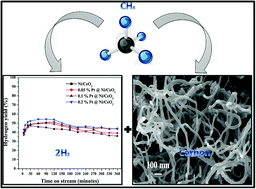
The catalytic decomposition of methane is a strategic process to produce COx-free hydrogen and carbon nanomaterials. In this work, for the first time, highly porous ceria was prepared by a urea-assisted solid state combustion method and used as a support for the preparation of a set of platinum-promoted nickel catalysts. The amount of Pt varied from 0.05% to 0.2% while retaining 20% nickel in the catalysts. The prepared catalysts were completely characterized for their structural, textural and redox properties, and their catalytic performance was tested for undiluted methane decomposition. A fine surface dispersion of nickel oxide over ceria was observed in the prepared catalysts. No peaks related to Pt were observed in the catalysts, indicating its good surface dispersion. An enhancement was observed in the properties of the Ni/CeO2 catalyst after the addition of Pt as a promoter. The crystallinity of NiO was not altered by the addition of Pt, whereas the specific surface area of the catalysts was increased with the incremental addition of Pt. Furthermore, the reduction temperature of nickel oxide was shifted to low temperature in the Pt-promoted catalysts due to the hydrogen spillover effect. The surface composition and chemical states of the Pt-promoted Ni/CeO2 catalysts were further studied using XPS. The Pt-promoted Ni/CeO2 catalysts exhibited high catalytic efficiency for methane decomposition due to their improved catalytic properties. The addition of Pt increased the hydrogen yield, and a significant increase in the hydrogen yield was observed for the incremental amount of Pt in the catalysts. Moreover, by increasing the reaction temperature from 650 °C to 750 °C, the hydrogen yield increased significantly. A maximum hydrogen yield of 65% was observed for the 0.2% Pt@Ni/CeO2 catalyst at 750 °C. The enhanced activity and stability of the catalysts were attributed to the synergistic effects of Ni and Pt due to their fine surface dispersion over ceria with a proper metal–support interaction. Multiwalled carbon nanotubes with different diameters were deposited over the catalysts. The carbon nanotubes deposited over the Ni/CeO2 catalyst were found to be more homogeneous than the Pt-promoted catalysts. Moreover, a wide hollow channel was observed in the carbon nanotubes deposited over the Pt-promoted catalyst.


 Please wait while we load your content...
Please wait while we load your content...