Ultrasensitive and highly selective FRET aptasensor for Hg2+ measurement in fish samples using carbon dots/AuNPs as donor/acceptor platform
Abstract
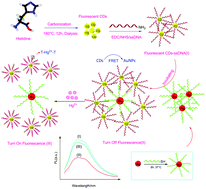
In this study, a novel fluorescence resonance energy transfer (FRET) aptasensor for detection of Hg2+ ions in real samples is described. The sensor is based on single-stranded DNA (ssDNA)-modified carbon dots (CDs) as an energy donor and complementary DNA (cDNA)-modified Au nanoparticles (AuNPs) as an energy acceptor. The CDs were synthesized by the hydrothermal reaction of histidine. The resulting CDs were conjugated with ssDNA through amidation reaction. The AuNPs-cDNA sample was prepared by self-immobilization of thiolated cDNA on the surface of Au nanoparticles. Specific hybridization between ssDNA and cDNA was achieved by mixing CDs-ssDNA and AuNPs-cDNA. The result of this phenomenon is quenching of the fluorescence associated with the FRET process. The response of the aptasensor was based on selective interaction of Hg2+ ions with thymine (T) groups in CDs-ssDNA, which led to displacement of AuNPs-cDNA by Hg2+ and an increase in fluorescence intensity. The aptasensor could be used in the range from 1.3 × 10−12 to 2.4 × 10−5 M for Hg2+ ions with a detection limit of 7.5 × 10−13 M. It showed high selectivity for Hg2+ with respect to several common metal ions. This aptasensor has been used for the determination of mercury ion concentration in fish samples.


 Please wait while we load your content...
Please wait while we load your content...