NaOH-promoted reaction of 1,1-dihaloalkenes and 1H-azoles: synthesis of dihetaryl substituted alkenes†
Abstract
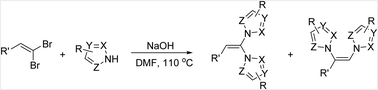
An efficient NaOH-promoted synthesis of dihetaryl substituted alkenes from 1,1-dihaloalkenes and 1H-azoles under mild conditions has been developed. The reaction occurred in moderate to good yields and tolerated aliphatic, heterocyclic, and aryl substituted 1,1-dihaloalkenes containing functionalities such as nitriles, ethers, and halogens. Imidazoles and triazoles also afforded the desired products.


 Please wait while we load your content...
Please wait while we load your content...