A high temperature reversible phase transition in a supramolecular complex of 15-crown-5 with tetraphenylboron sodium†
Abstract
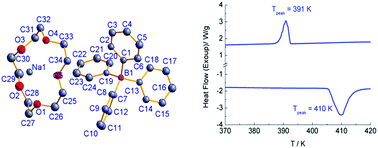
A supramolecular crystal, [Na(15-crown-5)][BPh4] (1), (15-crown-5 = 1,4,7,10,13-pentaoxacyclopentadecane, NaBPh4 = sodium tetraphenylboron), has been obtained by mixing the ethanol solution of 15-crown-5 and NaBPh4 in the molar ratio of 1 : 1. The crystal structure was determined at 293 K, revealing that two [Na(15-crown-5)]+ cations form a supramolecular dimer via sharing one side-edge of coordination pentagonal pyramids; also, there are significant H-bonding interactions between anions and supramolecularly dimeric cations. Differential scanning calorimetry (DSC) showed that 1 undergoes a reversible first-order phase transition at ca. 391 K (Tc) upon heating, with a thermal hysteresis of 19 K. ΔH and ΔS were estimated to be 6.9 kJ mol−1 and 17.7 J mol−1 K−1, respectively, in the heating run. The variable-temperature powder X-ray diffraction and dielectric spectra were collected, and both disclosed no significant difference between the low- and high-temperature phases. These results suggest that the phase transition is an ordered–disordered type, which probably involves the change of anion configuration.


 Please wait while we load your content...
Please wait while we load your content...